ISSUE #19 ABDR NEWSLETTER
April 17, 2023
Welcome to the latest Australian Breast Device Registry (ABDR) update.
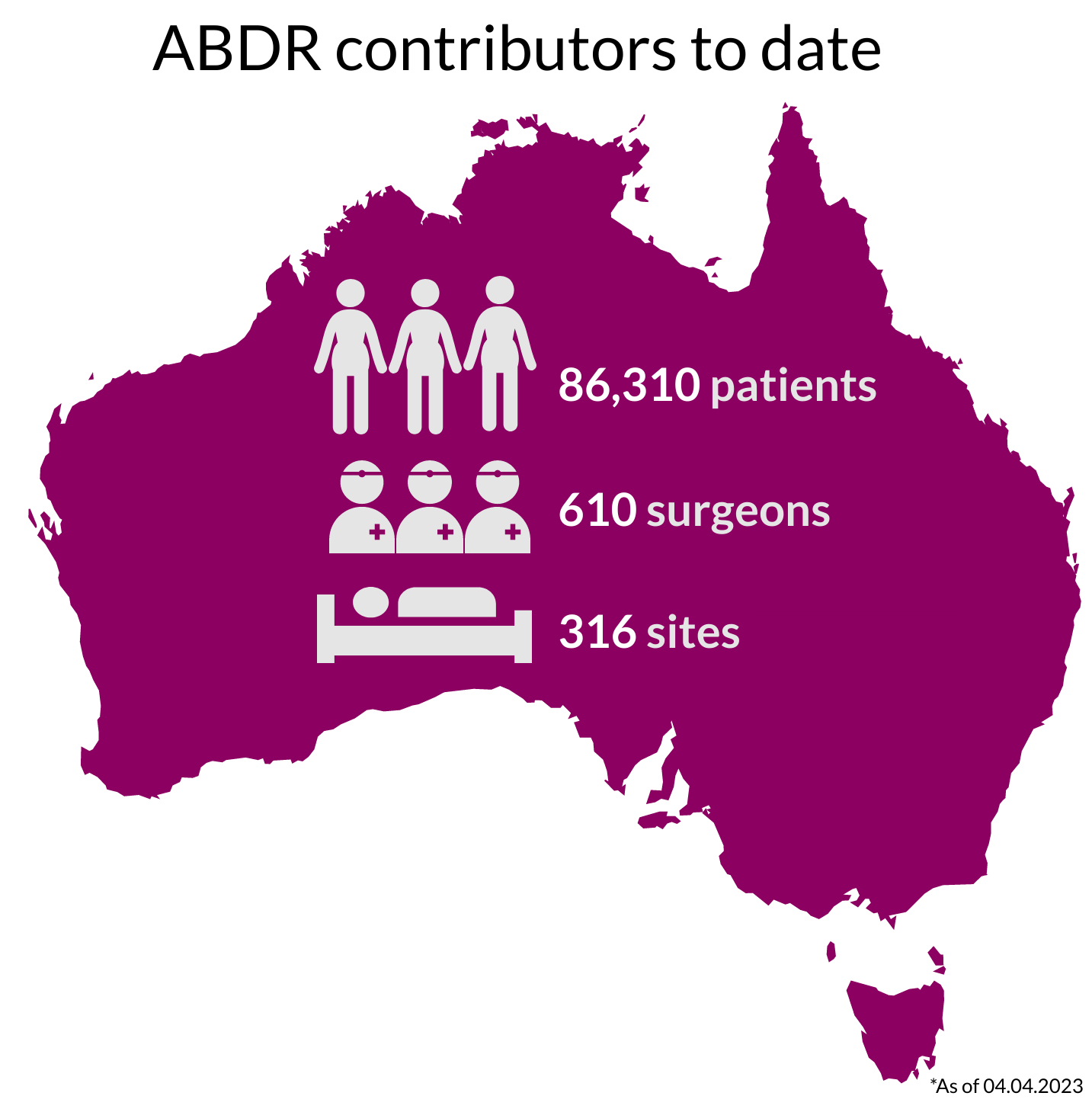
National Recruitment
Our thanks to Australians undergoing breast device surgery, their surgeons, and health care facilities, who continue to support the work of the ABDR. View 300+ participating sites here.
ABDR Data Dictionary
Late last month, we added to our website the new ABDR Data Dictionary to define each field of data captured on the Data Collection Form (DCF), as well as to explain how the data are sourced and entered into the registry’s database. This dictionary is particularly useful for researchers to understand the data fields captured by the ABDR, such as the types of devices and aspects of surgery. It is important to note that all data included in analyses and public reports arising from the ABDR are aggregated and do not contain any identifiable information about individual patients. Requests to analyse registry data must first demonstrate approval from a Human Research Ethics Committee.
Patient Leaflets
Because the ABDR is an OPT-OUT registry, every patient must be informed prior to surgery that their data will be sent to the ABDR. We recommend surgeons include this Patient Information Leaflet in the pre-surgery pack. Following surgery, ABDR staff will send each patient a more detailed Explanatory Statement. Patients are welcome to receive a copy of the statement prior to deciding to participate in the registry by contacting our team.
ABDR hosts international visitor
 Welcome Dr Juliet Vrolijk (left), a PhD candidate at the Dutch Breast Implant Registry, who has been with the ABDR for the last three months to write the final chapter of her thesis on the safety of breast implants on an international scale.
Welcome Dr Juliet Vrolijk (left), a PhD candidate at the Dutch Breast Implant Registry, who has been with the ABDR for the last three months to write the final chapter of her thesis on the safety of breast implants on an international scale.
The ABDR has a long history of international collaboration with breast device registries and is a member of the International Collaboration on Breast Registry Activities (ICOBRA).