ISSUE #22 ABDR NEWSLETTER
March 5, 2024
Welcome to the latest Australian Breast Device Registry (ABDR) update.
National Recruitment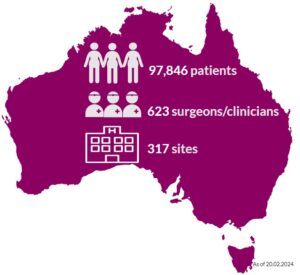
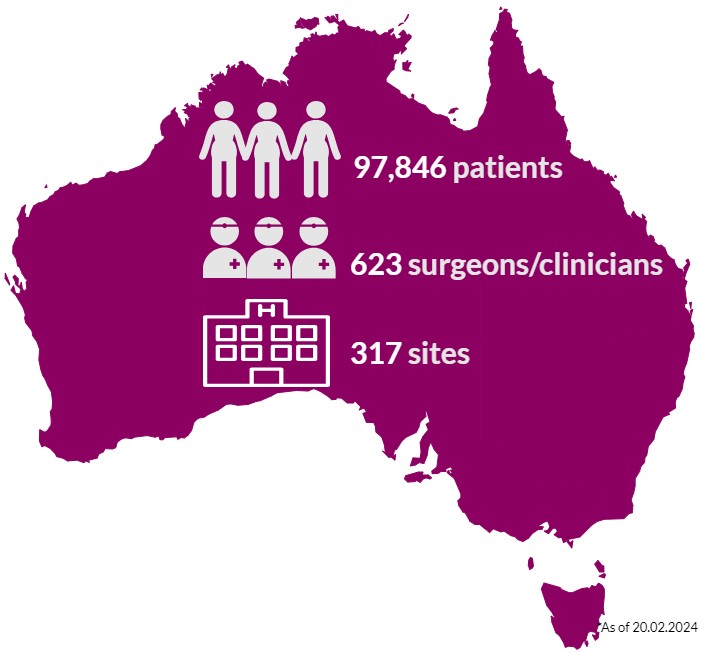
Almost 98,000 Australians who have had breast device surgery are in the ABDR. These patients may have had one or a combination of procedures, including breast device insertion, revision surgery, or device removal (explant). Our sincere thanks to patients, their surgeon/clinician, and health care facilities, who continue to support the work of the ABDR. Your contribution matters. View 300+ participating sites here.
ABDR 2023 Annual Report progress
We’ve put out a call for surgeons/clinicians and staff at health care facilities, such as theatre staff, to return completed registry data collection forms to the ABDR by 15th March 2024. This deadline aims to capture data from operations prior 2024, for analyses in the ABDR 2023 annual report, which is due for release later this year.
ABDR captures revision and removal (explant) procedures
Details of revision surgeries and/or removal of breast devices – implants, tissue expanders and mesh-like products – are critical to help the ABDR track the safety and performance of breast devices following insertion. The ABDR aims to identify and report on possible trends and complications associated with breast device surgery, such as rates of revision surgery. Surgeons and clinicians are encouraged to record revision and explant data on ABDR Data Collection Forms.
Patient Leaflets
As an OPT-OUT registry, patients must be informed prior to surgery that their data will be sent to the ABDR. We recommend surgeons/clinicians include this Patient Information Leaflet in the pre-surgery pack. Following surgery, ABDR staff will send each patient a more detailed Explanatory Statement. Patients are welcome to receive a copy of the statement prior to deciding to participate in the registry by contacting our team. Patient Information Leaflets can be ordered by emailing abdr@monash.edu